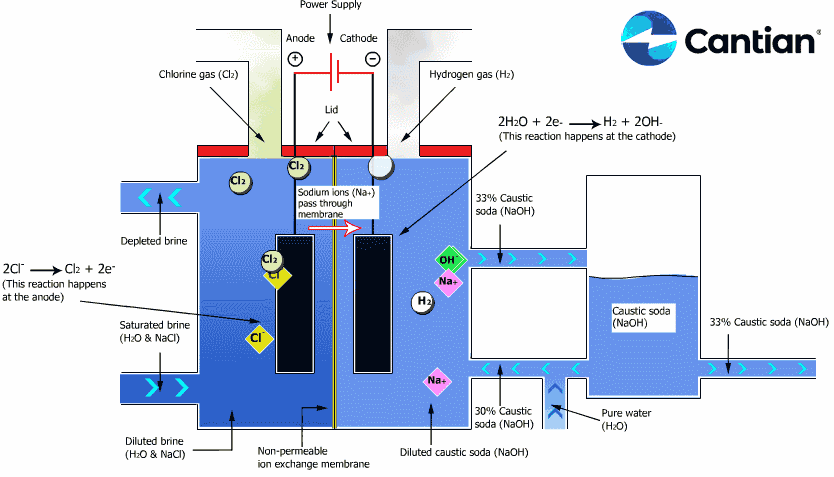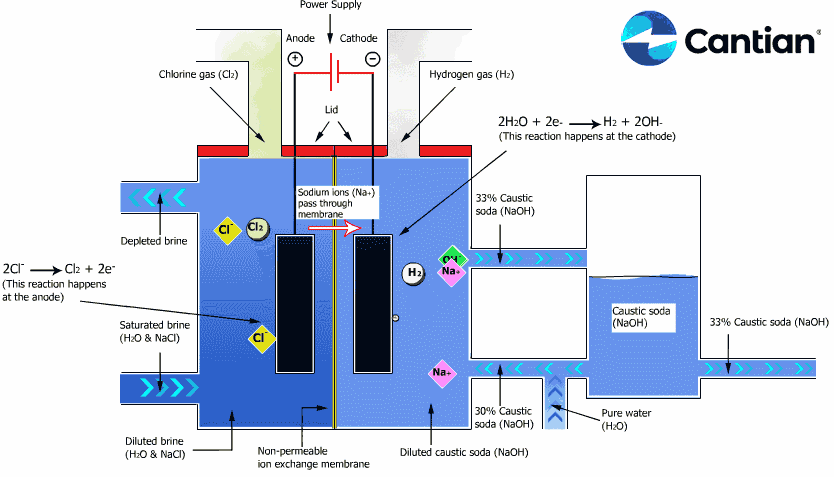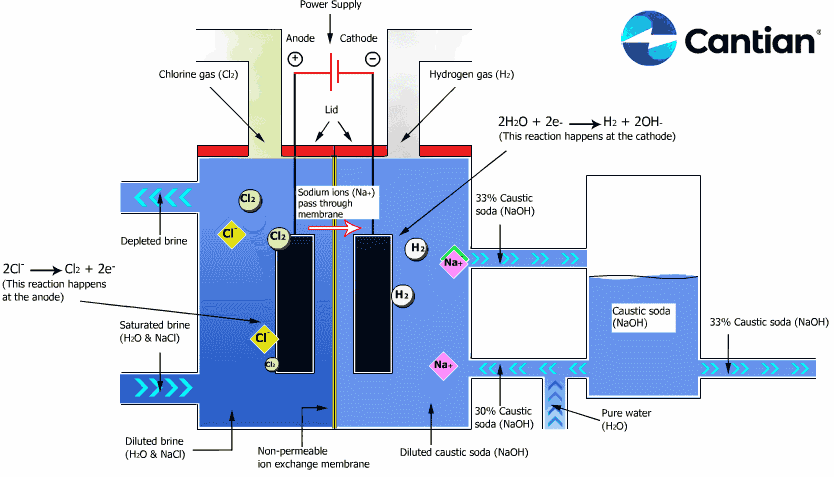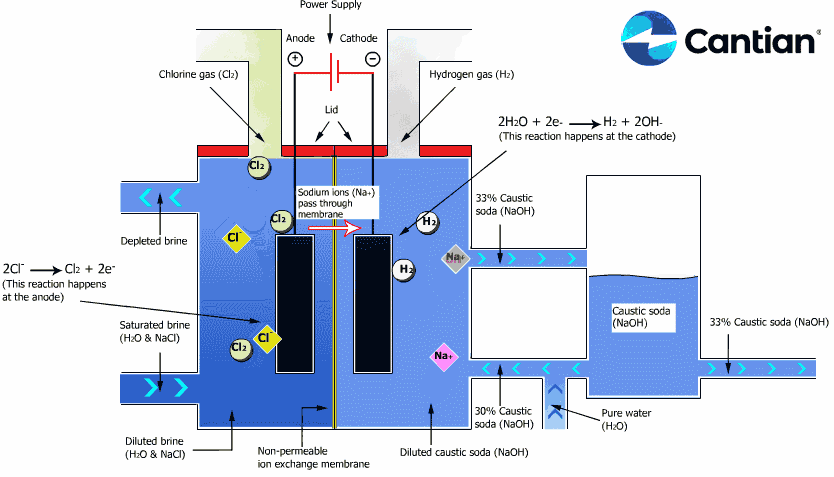
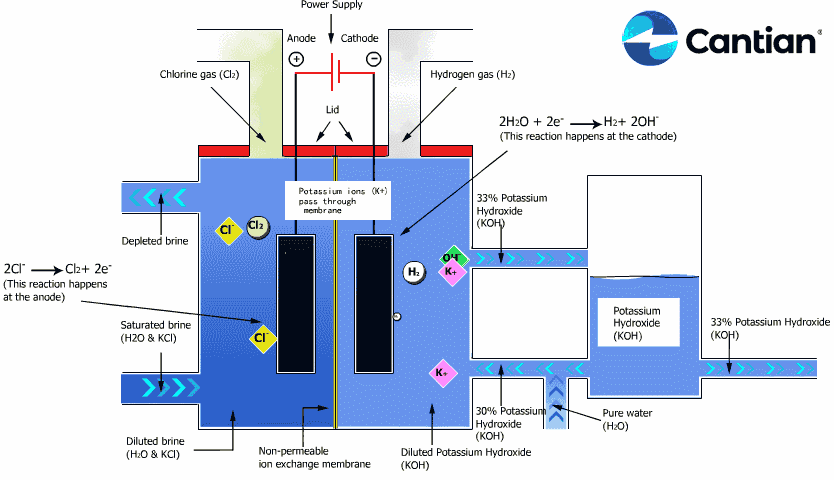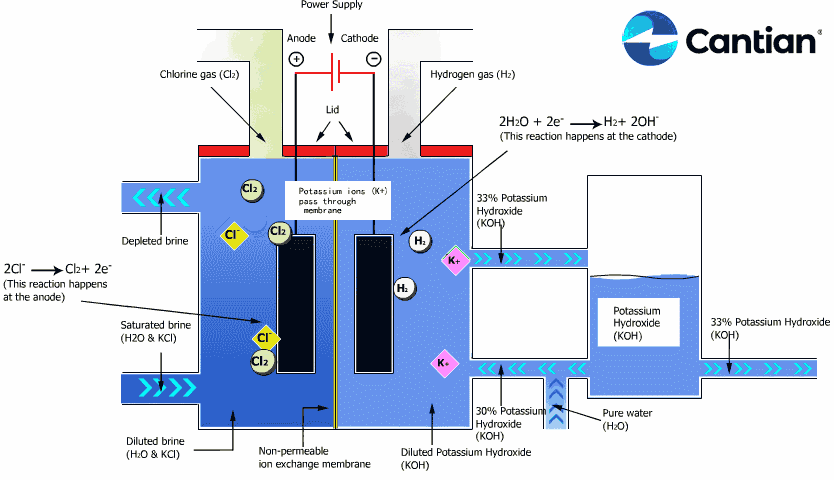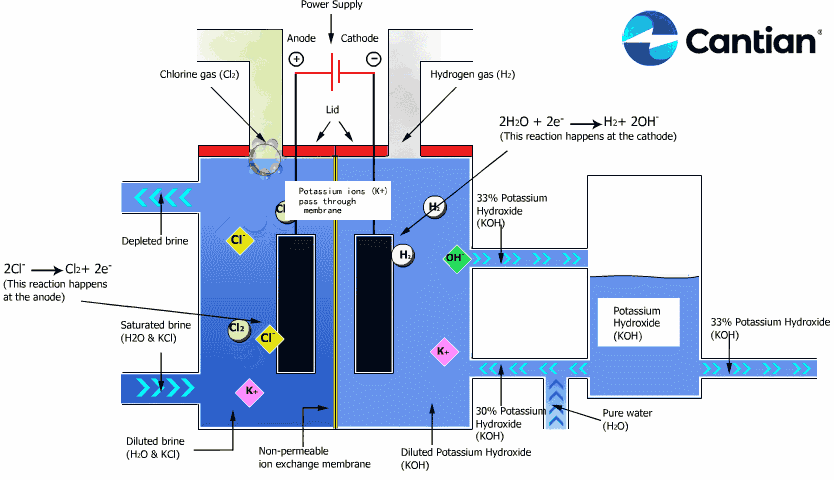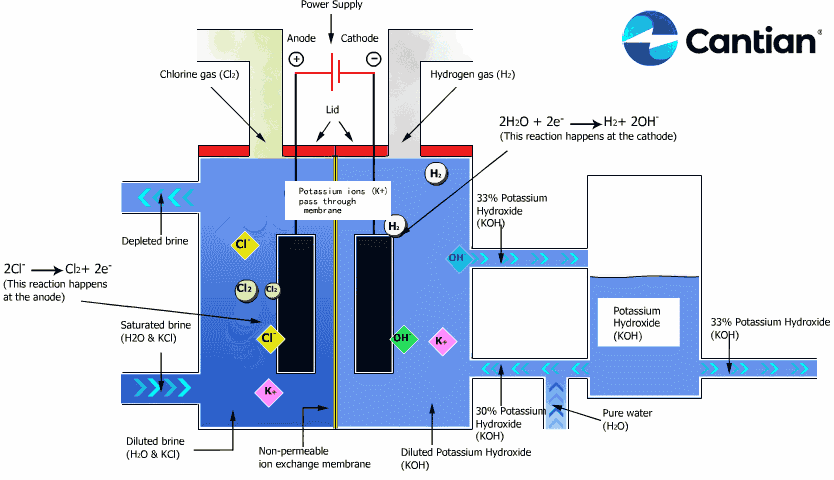
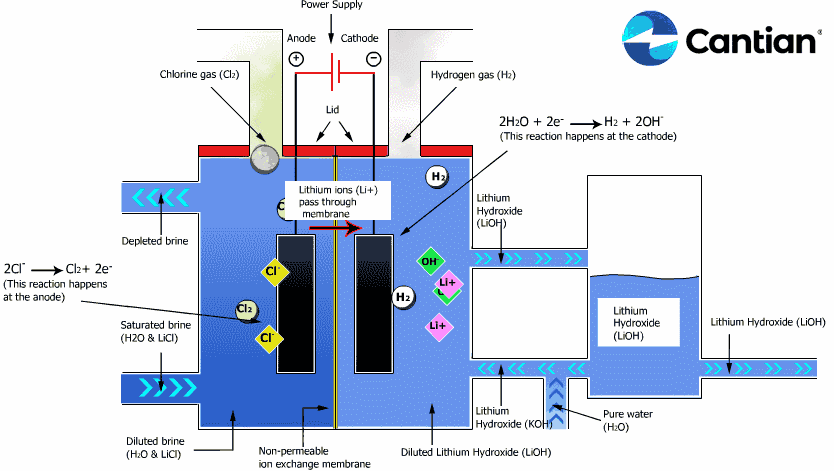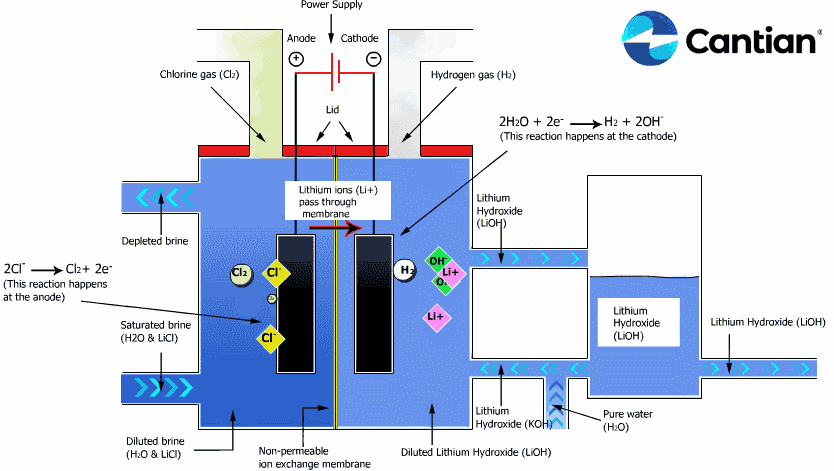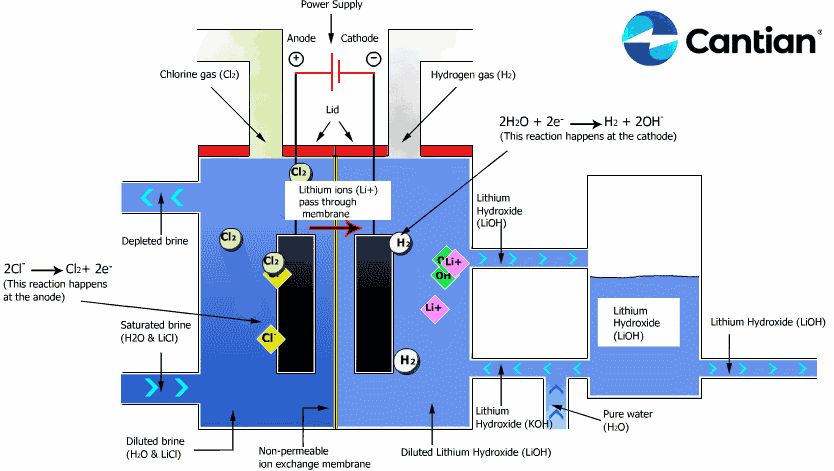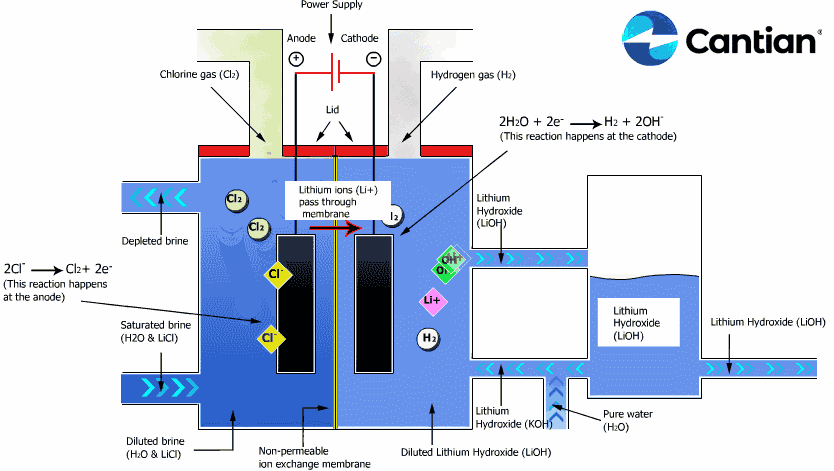
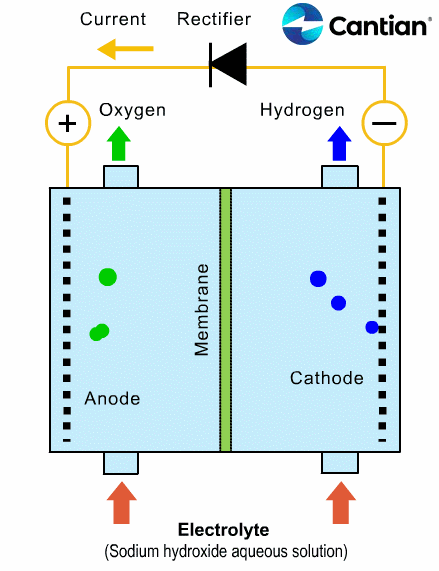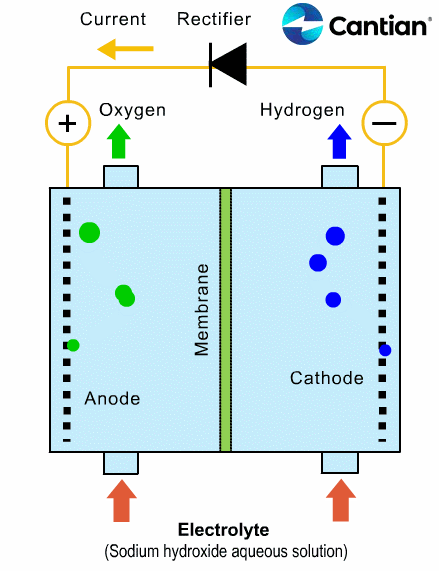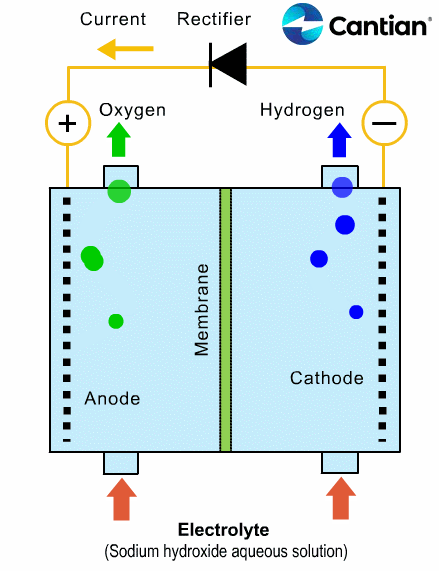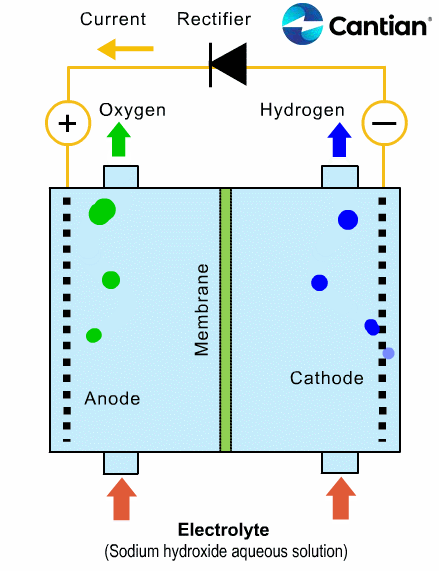
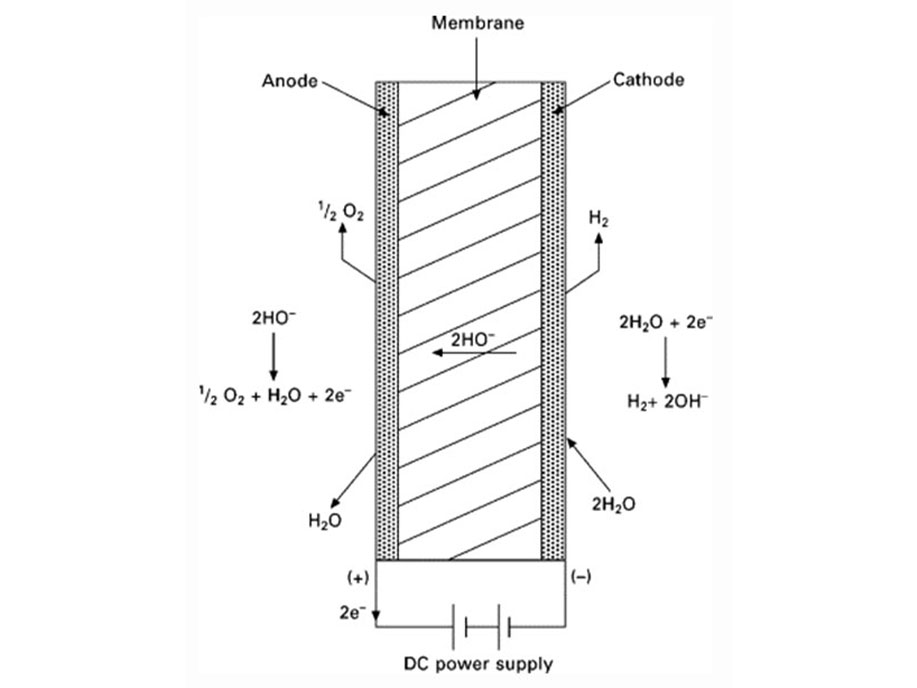
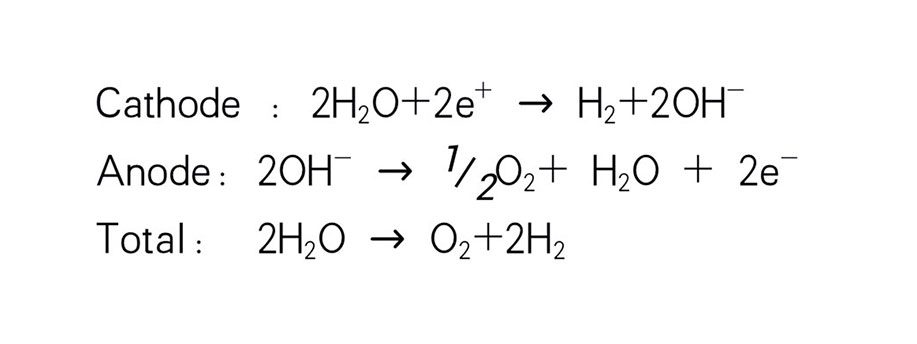As shown in the figure on the right, chlorine, caustic soda and hydrogen are produced by electrolyzing sodium chloride solution with the electricity applied to the solution through the electric terminals while sodium chloride solution and water are supplied to the cathodic and anodic chambers, respectively, in the ion-exchange membrane method.
As the anodic chamber is filled with sodium chloride solution, there are sodium ions (Na+) and chloride ions (Cl-) in the chamber. When electricity is applied to the solution, movement of the ions will occur. Since Na+ ions are cations, they will move from the anodic chamber, through the membrane and into the cathodic chamber, while Cl- ions will remain in the anodic chamber, since they are anions. Then, they will move to the anode, release electrons and become chlorine gas (Cl2) on the anode.
Meanwhile, part of water supplied into the cathodic chamber has been broken down to hydrogen ions (H+) and hydroxide ions (OH-). When electricity is applied to the solution, hydrogen ions will move to the cathode, acquire electrons on the cathode and become hydrogen gas (H2). Meanwhile, the hydroxide ions will move toward the anodic chamber. However, their movement will be blocked by the ion-exchange membrane and they will remain in the cathodic chamber with the sodium ions which have moved from anodic chamber. As a consequence, there will be a solution of caustic soda (NaOH) generated in the cathodic chamber.